The human body is a complex mosaic of tissues and organs, each aging at its own pace. Recent breakthroughs in single-cell sequencing have enabled scientists to construct a detailed Atlas of Senescent Cells, revealing striking variations in how different organs deteriorate over time. This biological cartography doesn’t just catalog where aging occurs—it exposes why some systems fail decades before others.
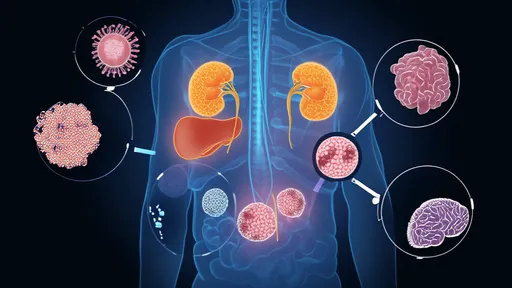
Deep within our cells, the molecular signatures of aging are anything but uniform. Researchers analyzing liver, heart, and kidney tissues discovered that cellular senescence markers appear 40% more frequently in certain organs compared to others. The kidneys showed particularly accelerated aging patterns, with accumulated DNA damage exceeding predictions by nearly two-fold. Meanwhile, the pancreas displayed unexpected resilience, maintaining functional cell populations well into advanced age.
What makes this atlas revolutionary isn’t just its precision—it’s the revelation that chronological age often poorly reflects biological reality. Two individuals at 65 may have cardiovascular systems differing by what amounts to 15 biological years. Such disparities explain why some octogenarians run marathons while others struggle with mobility decades earlier. The research underscores that aging isn’t a single process but thousands of micro-processes unfolding at varying speeds.
Blood vessels emerged as unexpected protagonists in the aging drama. Endothelial cells lining arteries exhibited some of the most pronounced inflammatory signatures, suggesting vascular aging may drive systemic decline. This finding dovetails with clinical observations that cardiovascular health predicts longevity more reliably than virtually any other biomarker. The data implies that protecting blood vessel integrity could disproportionately extend healthspan.
Sex differences in cellular aging patterns proved far more significant than anticipated. Female liver cells retained detoxification capabilities significantly longer than male counterparts, while male muscle tissue showed superior mitochondrial maintenance. These biological trade-offs may explain variations in disease susceptibility and lifespan between genders. The findings challenge one-size-fits-all approaches to anti-aging interventions.
Perhaps the most practical insight lies in the atlas’s predictive power. By mapping which cell types decline first in each organ, scientists can develop early warning systems for age-related diseases. Kidney specialists already use these profiles to detect pre-symptomatic renal decline up to eight years before standard diagnostics would catch it. Similar applications are emerging for neurodegenerative and cardiovascular conditions.
The immune system’s role as both victim and perpetrator of aging became starkly apparent. Senescent immune cells not only lose protective functions but actively secrete inflammatory compounds that accelerate tissue damage. This creates vicious cycles where aging immune cells hasten the aging of other systems—particularly evident in brain tissue where microglial dysfunction precedes cognitive decline.
Surprisingly, the skin—long considered a mere outward indicator of aging—showed profound connections to systemic health. Fibroblast senescence patterns correlated strongly with internal organ aging, suggesting skin biopsies might someday serve as windows to overall biological age. Such accessible biomarkers could revolutionize preventive medicine by enabling routine aging assessments during standard checkups.
As this cellular cartography grows more detailed, it’s rewriting fundamental concepts about human longevity. The traditional view of aging as gradual, uniform deterioration has given way to a dynamic model where organs influence each other’s decline through complex signaling networks. This paradigm shift opens avenues for targeted interventions that could decouple chronological aging from biological dysfunction.
The atlas doesn’t just document aging—it illuminates pathways to disrupt it. Researchers identified several cell populations that resist senescence exceptionally well, offering clues for therapeutic development. These biological outliers, comprising about 3% of studied cells, maintain youthful gene expression patterns despite their advanced chronological context. Harnessing their secrets could lead to breakthroughs in regenerative medicine.
Ethical considerations loom large as this technology progresses. The ability to quantify organ-specific aging raises questions about health disparities, insurance implications, and even personal identity when biological age diverges significantly from calendar age. Society will need frameworks to ensure these powerful diagnostics benefit populations equitably rather than exacerbating existing inequalities.
Commercial applications are advancing faster than many anticipated. Three biotechnology startups have licensed atlas data to develop organ-specific aging clocks, while major pharmaceutical companies are screening compounds that might selectively rejuvenate particular cell types. The first clinical trials targeting senescent kidney cells could begin within 18 months, marking a new era of precision geroscience.
Critically, the research validates that aging interventions needn’t target the whole body simultaneously. Slowing deterioration in just one pivotal organ system—whether the vasculature, immune system, or liver—could produce cascade effects delaying overall decline. This approach makes anti-aging strategies more pharmacologically feasible than comprehensive cellular rejuvenation.
The atlas remains incomplete, with neural tissues and the gastrointestinal system representing the next mapping frontiers. Early neural data suggests brain aging follows entirely different rules than peripheral tissues, with some neurons showing negligible molecular changes over decades while others deteriorate rapidly. These nuances will prove vital for tackling neurodegenerative diseases.
Ultimately, this project represents more than scientific achievement—it’s a philosophical recalibration. By exposing aging as a constellation of processes rather than a monolithic event, the work empowers targeted interventions that could transform what it means to grow old. The organ aging maps don’t just tell us where time leaves its mark; they reveal where we might erase it.
By /Jul 3, 2025

By /Jul 3, 2025

By /Jul 3, 2025

By /Jul 3, 2025
By /Jul 3, 2025

By /Jul 3, 2025

By /Jul 3, 2025
By /Jul 3, 2025

By /Jul 3, 2025
By /Jul 3, 2025
By /Jul 3, 2025

By /Jul 3, 2025
By /Jul 3, 2025

By /Jul 3, 2025
By /Jul 3, 2025

By /Jul 3, 2025
By /Jul 3, 2025
By /Jul 3, 2025
By /Jul 3, 2025
By /Jul 3, 2025