The quest for an HIV cure has entered a pivotal phase as researchers focus their efforts on targeting viral reservoirs – the hidden sanctuaries where the virus evades current treatments. These reservoirs, composed of long-lived immune cells harboring dormant HIV DNA, represent the final frontier in the battle to eradicate the infection completely. While antiretroviral therapy (ART) can suppress viral replication to undetectable levels, it cannot eliminate these stubborn reservoirs that reignite the infection whenever treatment stops.
Understanding viral reservoirs requires examining their biological complexity. When HIV infects CD4+ T-cells, it integrates its genetic material into the host cell's DNA. Most infected cells die quickly, but a small fraction enter a resting state, becoming memory T-cells designed to persist for decades. These latently infected cells neither produce viral particles nor display viral proteins on their surface, making them invisible to both the immune system and antiretroviral drugs. The randomness of viral integration creates unique reservoir signatures in each patient, further complicating eradication efforts.
Recent advances in reservoir detection have revolutionized the field. Ultra-sensitive assays can now identify single copies of HIV DNA hiding among billions of cells. Techniques like the "Full-Length Individual Proviral Sequencing" (FLIPS) method allow scientists to distinguish between intact viruses capable of rebounding and defective viral fragments. This precision mapping reveals that only about 5-10% of reservoir cells contain replication-competent virus, yet this small fraction poses an insurmountable barrier to cure efforts.
The shock and kill strategy has emerged as a leading approach to reservoir clearance. This two-step process involves first reactivating dormant HIV (the "shock") to force reservoir cells to produce viral proteins, then eliminating these exposed cells through immune attack or viral cytopathic effects (the "kill"). Latency reversing agents like histone deacetylase inhibitors show promise in laboratory studies, but clinical trials have yielded mixed results – successfully exposing reservoir cells without achieving meaningful reductions in reservoir size.
Parallel approaches aim to make reservoir cells self-destruct. "Block and lock" strategies seek to permanently silence HIV proviruses using epigenetic modifiers, while gene editing tools like CRISPR-Cas9 attempt to surgically remove integrated HIV DNA. The Berlin and London patients, cured through stem cell transplants from CCR5Δ32 donors, demonstrated that reservoir elimination is possible, albeit through impractical and risky procedures for widespread use.
Exciting developments in immune therapies are reshaping the reservoir clearance landscape. Bispecific antibodies engineered to simultaneously bind HIV-infected cells and cytotoxic T-cells have shown remarkable efficiency in eliminating reservoir cells in animal models. Therapeutic vaccines designed to boost HIV-specific immune responses may provide the necessary "kill" mechanism to complement latency reversal. Meanwhile, engineered T-cell receptors that recognize conserved HIV epitopes offer another pathway to target diverse reservoir variants.
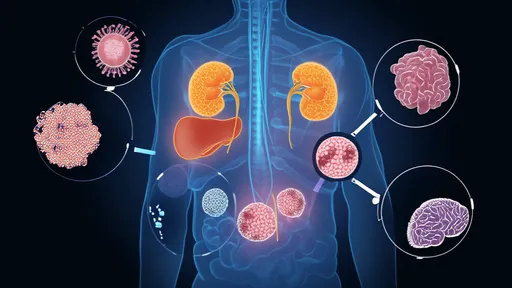
The challenge of tissue reservoirs adds another layer of complexity. Beyond blood, HIV persists in lymphoid tissues, the central nervous system, and gut-associated lymphoid tissue – anatomical sanctuaries with distinct microenvironmental conditions. The blood-brain barrier prevents many drugs from reaching viral reservoirs in the brain, while the unique immune environment of gut tissues may foster different reservoir dynamics. Successful eradication strategies must account for these geographical variations in viral persistence.
Measuring progress in reservoir reduction presents its own challenges. Current assays cannot detect extremely small reservoirs that might still cause rebound, while the stochastic nature of viral reactivation means prolonged analytical treatment interruptions remain the gold standard for assessing cure potential. Biomarker development lags behind therapeutic innovation, creating a critical bottleneck in evaluating new interventions.
As research advances, ethical considerations grow more prominent. Analytical treatment interruptions necessary for cure research carry risks of viral rebound and potential transmission. Participants in cure studies often face difficult decisions between contributing to scientific progress and maintaining viral suppression through continuous ART. The field must balance urgency with caution as experimental approaches move into human trials.
The economic dimensions of cure research cannot be overlooked. While ART has become more accessible globally, the infrastructure required for reservoir-targeting therapies – including advanced diagnostics and specialized care – may create new disparities in access. Successful cure strategies will need to balance efficacy with scalability to benefit the 38 million people living with HIV worldwide.
Looking ahead, combination approaches appear most promising for reservoir clearance. No single intervention has yet demonstrated sufficient potency to eliminate all replication-competent HIV. Future strategies will likely integrate latency reversal with enhanced immune effectors, gene editing, and possibly therapeutic vaccination. The path to a cure remains uncertain, but the growing understanding of viral reservoirs has transformed what was once considered impossible into a tractable scientific challenge.
Each new discovery about reservoir biology refines the search for vulnerabilities. From the identification of specific biomarkers marking reservoir cells to the development of agents that selectively target these cells without global immune activation, the toolkit for reservoir clearance continues to expand. While formidable obstacles remain, the scientific community's focused attention on viral reservoirs has brought the prospect of an HIV cure closer to reality than ever before.
By /Jul 3, 2025

By /Jul 3, 2025

By /Jul 3, 2025

By /Jul 3, 2025
By /Jul 3, 2025

By /Jul 3, 2025

By /Jul 3, 2025
By /Jul 3, 2025

By /Jul 3, 2025
By /Jul 3, 2025
By /Jul 3, 2025

By /Jul 3, 2025
By /Jul 3, 2025

By /Jul 3, 2025
By /Jul 3, 2025

By /Jul 3, 2025
By /Jul 3, 2025
By /Jul 3, 2025
By /Jul 3, 2025
By /Jul 3, 2025