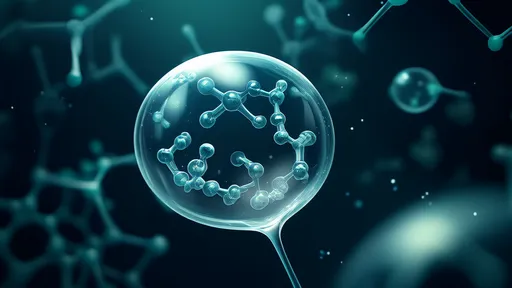
In the bustling metropolis of a living cell, where countless molecular interactions occur every second, scientists have discovered remarkable structures that defy traditional understanding of cellular organization. These protein droplets, once thought to be mere aggregates, are now recognized as sophisticated microreactors that orchestrate vital biochemical processes with exquisite precision.
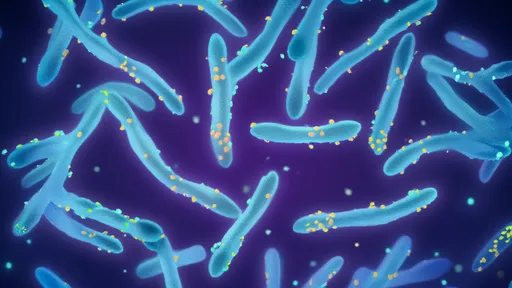
The discovery of membrane-less organelles formed through liquid-liquid phase separation has revolutionized our understanding of cellular compartmentalization. Unlike their membrane-bound counterparts such as mitochondria or the nucleus, these protein condensates form through spontaneous self-assembly, creating dynamic hubs for specialized chemistry. Researchers have observed how these droplets selectively concentrate specific molecules while excluding others, effectively creating tailored environments for biochemical reactions.
Nature's ingenious solution to cellular organization appears in these transient structures that form and dissolve according to cellular needs. The physical principles governing their formation share surprising similarities with everyday phenomena like oil droplets in vinegar or water condensing on a cold surface. Yet in the cellular context, this phase separation achieves remarkable functional sophistication. Proteins containing intrinsically disordered regions play crucial roles in this process, their flexible chains enabling rapid assembly and disassembly in response to environmental cues.
Recent breakthroughs in super-resolution microscopy have allowed scientists to observe these droplets in unprecedented detail. What emerges is a picture of astonishing complexity - some droplets appear to have internal structure themselves, with different regions specialized for distinct biochemical processes. Others demonstrate the ability to fuse and divide like living entities, exchanging components with their surroundings in a carefully regulated dance of molecular interactions.
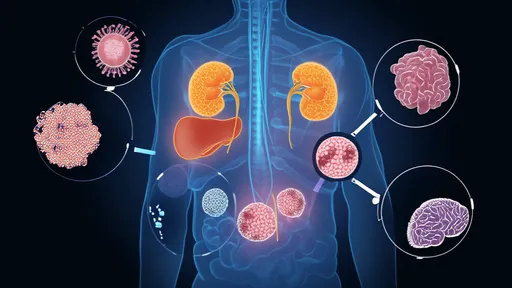
The implications for fundamental biological processes are profound. These protein droplets appear involved in everything from gene expression to stress response. In the nucleus, they concentrate transcription factors and nucleic acids to regulate gene activity. During cellular stress, they sequester damaged proteins and mobilize repair mechanisms. Their malfunction has been implicated in neurodegenerative diseases, suggesting these dynamic structures play critical roles in both health and disease.
What makes these droplets particularly fascinating is their ability to maintain distinct chemical environments without physical barriers. Through a combination of weak molecular interactions and selective permeability, they achieve functional compartmentalization while remaining highly dynamic. This challenges our traditional view of cellular organization and suggests evolution has harnessed the physics of phase separation to create versatile, responsive reaction vessels.
Engineering applications inspired by these natural microreactors are already emerging. Synthetic biologists are designing artificial protein droplets that could perform specialized chemical reactions in industrial settings. Pharmaceutical researchers are exploring how to target disease-related droplets for therapeutic intervention. The potential to create programmable biomolecular condensates opens new frontiers in biotechnology and materials science.
As research progresses, scientists are uncovering an intricate regulatory network that controls droplet formation and function. Post-translational modifications, molecular chaperones, and even mechanical forces all contribute to maintaining the delicate balance between order and fluidity that characterizes these structures. This regulatory complexity suggests cells have evolved sophisticated mechanisms to harness phase separation while preventing its potentially harmful consequences.
The study of protein droplets bridges disciplines from physical chemistry to cell biology, requiring innovative approaches to understand their paradoxical nature - stable yet dynamic, organized yet fluid. Advanced computational models combined with cutting-edge experimental techniques are revealing how cells use these structures to optimize biochemical processes, respond to environmental changes, and perhaps even encode information in their physical state.
Looking ahead, researchers anticipate these findings will transform our understanding of cellular organization. The traditional dichotomy between structured organelles and the supposedly disorganized cytosol gives way to a new paradigm where transient, functional compartments form and dissolve according to cellular needs. This dynamic view of cellular organization may hold the key to understanding how cells achieve such remarkable efficiency and adaptability in their biochemical operations.
From fundamental biology to potential therapeutic applications, the exploration of protein droplets as cellular microreactors represents one of the most exciting frontiers in modern life sciences. As we continue to unravel their secrets, we may find that these ephemeral structures play even more central roles in the complex chemistry of life than we currently imagine.
By /Jul 3, 2025

By /Jul 3, 2025

By /Jul 3, 2025

By /Jul 3, 2025
By /Jul 3, 2025

By /Jul 3, 2025

By /Jul 3, 2025
By /Jul 3, 2025

By /Jul 3, 2025
By /Jul 3, 2025
By /Jul 3, 2025

By /Jul 3, 2025
By /Jul 3, 2025

By /Jul 3, 2025
By /Jul 3, 2025

By /Jul 3, 2025
By /Jul 3, 2025
By /Jul 3, 2025
By /Jul 3, 2025
By /Jul 3, 2025