The intricate dance of biological rhythms extends far beyond the well-known sleep hormone melatonin, revealing a sophisticated genetic network that orchestrates our internal clocks. While melatonin has long been the poster child for circadian regulation, scientists are now uncovering a vast array of clock genes that work in concert to fine-tune our physiological processes. These genes form a complex web of interactions, influencing everything from metabolism to cognition, and their discovery is reshaping our understanding of how organisms adapt to the Earth's daily rotations.
At the heart of this genetic timekeeping system lies the transcriptional-translational feedback loop (TTFL), a core mechanism that drives circadian oscillations. Central to this loop are the genes CLOCK and BMAL1, which activate the expression of other clock components, including PER and CRY. As these proteins accumulate, they eventually inhibit CLOCK and BMAL1, creating a self-sustaining cycle that repeats approximately every 24 hours. This elegant molecular ballet ensures that our internal rhythms remain synchronized with the external environment.
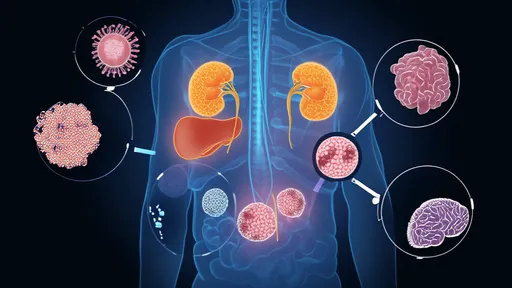
Emerging research highlights the remarkable tissue specificity of these clock genes. While the suprachiasmatic nucleus (SCN) in the hypothalamus acts as the master pacemaker, peripheral clocks in organs like the liver, heart, and kidneys exhibit their own rhythmic expression patterns. This decentralized system allows different tissues to optimize their functions according to local demands while maintaining overall coordination. The liver's clock, for instance, regulates metabolic enzymes to anticipate feeding times, demonstrating how peripheral oscillators contribute to whole-body homeostasis.
The influence of clock genes extends into unexpected physiological territories. Recent studies have connected circadian gene variants to variations in human chronotypes, explaining why some individuals naturally wake at dawn while others thrive at midnight. Beyond sleep-wake patterns, these genetic factors appear to affect drug metabolism efficacy, cardiovascular function, and even mood regulation. The NR1D1 gene, a crucial component of the clock network, has been implicated in both metabolic disorders and depression, illustrating the far-reaching consequences of circadian disruption.
Environmental challenges to our biological clocks have never been greater. Artificial light exposure, shift work, and erratic eating patterns constantly perturb the delicate balance of clock gene expression. Scientists are particularly concerned about how chronic circadian misalignment might contribute to the modern epidemics of obesity, diabetes, and cancer. The REV-ERBα protein, another key clock component, has been shown to regulate both lipid metabolism and tumor growth, providing a molecular link between disrupted rhythms and disease pathogenesis.
Exciting therapeutic possibilities are emerging from our growing understanding of clock genetics. Chronopharmacology—the timed administration of medications according to circadian principles—is demonstrating improved efficacy and reduced side effects for various treatments. Researchers are also developing small molecules that target specific clock proteins, offering potential interventions for metabolic and sleep disorders. These advances highlight how deciphering the clock gene network could revolutionize personalized medicine approaches.
The evolutionary conservation of clock genes across species underscores their fundamental importance. From fruit flies to humans, the core circadian machinery remains remarkably similar, suggesting these rhythms provided crucial adaptive advantages throughout evolutionary history. Contemporary research continues to uncover new layers of complexity, including post-translational modifications, epigenetic regulation, and intricate cross-talk with other signaling pathways that modulate clock function.
As we peel back the layers of circadian biology, it becomes increasingly clear that melatonin represents just one piece of a much larger puzzle. The clock gene network forms an intricate biological computer that processes environmental time cues and coordinates appropriate physiological responses. Future research will likely reveal even more connections between these molecular timekeepers and various aspects of health, potentially offering new strategies for disease prevention and treatment through circadian optimization.
By /Jul 3, 2025

By /Jul 3, 2025

By /Jul 3, 2025

By /Jul 3, 2025
By /Jul 3, 2025

By /Jul 3, 2025

By /Jul 3, 2025
By /Jul 3, 2025

By /Jul 3, 2025
By /Jul 3, 2025
By /Jul 3, 2025

By /Jul 3, 2025
By /Jul 3, 2025

By /Jul 3, 2025
By /Jul 3, 2025

By /Jul 3, 2025
By /Jul 3, 2025
By /Jul 3, 2025
By /Jul 3, 2025
By /Jul 3, 2025